Chapter 2: Textual Exercises

Lairikpro
0 Comment
Q1, Define the term Valence Shell and Valence electrons.
Q2. Why do elements combine chemically?
Q3. What is an electrovalent bond and how is it formed?

Some other examples are: MgCl2, CaCl2, MgO, Na2S, CaH2, AlF3, NaH, KH, K2O, KI, RbCl, NaBr, CaH2 etc.
Q4. What is a covalent bond and how is it formed?
Ans: The covalent bonding is a type of bond which is caused by the mutual electrical attraction between the two positive nuclei of the two atoms forming the bond, and the SHARING of a pair of negative electrons between them.
Covalent Bonding can be Achieved in two Ways:






Ans: Valence shell is the outermost shell of every element. Atom of every element have different electronic configurations based on the atomic number of each element. Electronic configuration refers to the distribution of electrons in various shells/orbits/energy levels of every atom. For example,Sodium
Atomic no.=11
Electronic configuration- K L M : 2 8 1
Atomic no.=11
Electronic configuration- K L M : 2 8 1
As you can see sodium has three shells in it. The outermost shell of sodium is called the valence shell and the electrons in the valence shell are called valence electrons.
Q2. Why do elements combine chemically?
Ans: Elements combine together to complete their valence shell (except noble gases) and for gaining stability. Hence, they have to react with other elements to attend a stable configuration of nearest Noble gas.
Q3. What is an electrovalent bond and how is it formed?
Ans: Electrovalent bond is a type of chemical bond formed by the transfer of one or more electrons from one atom to another, so that oppositely charged ions are produced. For example, the bond between the sodium and chlorine atoms in sodium chloride (NaCl) is formed by the transfer of an electron from sodium to chlorine, creating Na+ and Cl– ions. The electrostatic attraction between these ions provides the bonding in NaCl.

Some other examples are: MgCl2, CaCl2, MgO, Na2S, CaH2, AlF3, NaH, KH, K2O, KI, RbCl, NaBr, CaH2 etc.
Q4. What is a covalent bond and how is it formed?
Ans: The covalent bonding is a type of bond which is caused by the mutual electrical attraction between the two positive nuclei of the two atoms forming the bond, and the SHARING of a pair of negative electrons between them.
In the formation of Covalent molecules, an atoms shares as many electrons with other atoms as needed to acquire nearest noble gas configurations (Octet or Duplet) i.e. This bond is formed by mutual sharing of electrons between the combining atoms to attain the stable noble gas configuration. Sharing one pair of electrons from a single bond, two pairs a double bond and the pairs a triple bond.
- Sharing of electrons between atoms of the same kind E.g. Formation of H2, Cl2, O2, etc.
- Sharing of electrons between atoms of different kind E.g. Formation of CH4, H2O, NH3, etc.

Formation of sodium fluoride (NaF): The transfer of an electron from a neutral sodium atom to a neutral fluorine atom creates two oppositely charge ions: Na+ and F–. Attraction of the oppositely charged ions is the ionic bond between Na and F.
Q5. How does an Ionic bond differ from a covalent bond? Illustrate your answer with two examples of each type of bond.
Ans:
Difference Between Ionic and Covalent Bond
| |
Covalent Bonds
|
Ionic Bonds
|
| A covalent bond is formed between two similar electronegative non-metals | This type of bond is formed between a metal and non-metal |
| Bonds formed from covalent bonding have a Definite shape | Ionic Bonds have No definite shape |
| Low Melting Point and Boiling Point | High Melting Point and Boiling Point |
| Low Polarity and more Flammable | High Polarity and less Flammable |
| Covalent Bonds are in Liquid or gaseous State at room temperature | At room temperature, Ionic Bonds have Solid-state. |
| Examples: Methane, Hydrochloric acid | Example: Sodium chloride, Sulfuric Acid |

Illustrative Examples: A. Ionic Bond:
1.Formation of sodium chloride (NaCl):
2.Formation of Magnesium Chloride (MgCl2):
B. Covalent Bond:

Q6. What is a polar molecule? Give examples.
Ans: A polar molecule is a molecule containing polar bonds where the sum of all the bond's dipole moments is not zero. Polar bonds form when there is a difference between the electronegativity values of the atoms participating in a bond. Polar molecules also form when the spatial arrangement of chemical bonds leads to a more positive charge on one side of the molecule than the other.
Examples of Polar Molecules
- Water (H2O) is a polar molecule. The bonds between hydrogen and oxygen are distributed so that the hydrogen atoms are both on one side of the oxygen atom rather than evenly spaced. The oxygen side of the molecule has a slight negative charge, while the side with the hydrogen atoms has a slight positive charge.
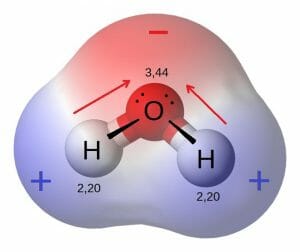
Q7. Give electron dot representation for the following molecules.
F2, HF and H2O
Ans:
F2, HF and H2O
Ans:

Q8. State four properties of each of electrovalent and covalent compounds.
Ans:
Four Properties of electrovalent compounds:- Crystal Structure: In the solid state of electrovalent compounds, anions and cations are arranged in a regular manner. This is a crystal in which anions are surrounded by a definite number of cations and vice-versa.
- Physical Nature: Ionic or electrovalent compounds are generally hard. Their hardness increases with increasing ionic charge and the decreasing distance between ions.
- Solubility: Positive ion of ionic compound attaches to the negative part of a polar solvent and negative ion of ionic compound attach with the positive part of the polar solvent. Therefore, ionic or electrovalent compounds are soluble in polar solvents like water and insoluble in non-polar solvents like benzene, ether, alcohol.
- Melting Point and Boiling Point: Electrovalent or ionic compounds have high melting and boiling points because they need a large amount of energy to break strong ionic bonds.
Four Properties of Covalent Compounds:
- Covalent compounds usually have low melting points. An exception to this include molecules of silica and diamonds that have a high melting point.
- These compounds have low boiling points. This can be attributed to their weak force of attraction between the various bonded atoms. Van Der Waals forces bind these atoms.
- These compounds are usually gases and liquids with low boiling and melting points.
- The solid covalent compounds have soft structures like graphite. This is because of the presence of a cloud of electrons in between each layer of carbons atoms.
- These compounds are non-conductors of electrical charge. The absence of charged ions is the main reason behind this. An exception to this is graphite, where we see a cloud of electrons. These make graphite a good conductor.
Q9. The elements w,x,y and z have atomic number 7,9,10 and 11 respectively. Wrtite the formula of the compound between the following pairs of elements and indicate the type of the bonding present using dot(.) and crosses (X) to represent valence electrons.
a) w and x b) x and x
c) w and z d) y and y
Ans: a) Here, atomic number of w =7
∴ its electronic configuration=2,5 & atomic number of x = 9

∴ its electronic configuration=2,5 & atomic number of x = 9
∴ its electronic configurations=2,7
Hence, the formula of the compound is wx3
Thus, the type of bonding is covalent bond. It is shown below:

Here, w has three electrons short to complete is octet. When it shares three of its electrons with x atoms, the stable electronic configuration of neon is achieved and three x-atoms also acquire the stable neon configuration. Thus, covalent compound (wx3) is formed.
b) Here, atomic number of x = 9
∴ its electronic configurations=2,7
the formula of the compound is x2 and the type of bonding is covalent bond. It is shown below:



c) Here, atomic number of w=7
∴ its electronic configuration=2,5
& atomic number of z=11
∴ its electronic configuration=2,8,1.
Hence, the formula of the compound is z3w & the type of bonding is electrovalent bond. The combination is given below:

d) Hence, atomic number of y=10
∴ its electronic configuration=2,8
Hence, the compound can not be formed beacuse eight electrons in the outermost shell of each atom makes the atom stable. It contains only one atom.


Post a Comment
Post a Comment