Chapter2: Let us answer these (page-28)

Lairikpro
0 Comment
Q1. Which electronic configuration favours inert or noble behavior of elements?
Ans: The electronic configuration with eight electrons (two electrons in case there is K-shell as valence shell) in the outermost shell favours the inert or noble behavior of elements.
Q2. Why do most of the elements form ions?
Ans: Elements that form ions do so because their natural electron configuration isn’t stable. By gaining or losing electrons they can attain a full valence shell, i.e. that of a noble gas, which is stable.
Elements that already have full shells, and smaller atoms that have half-filled shells, such as carbon and silicon, typically don’t form ions.
Q3. What kind of elements form cation? Support your answer by two example.
Ans: Metals or electropositive elements whose atoms can lose one or more electrons form positive ions |(or cations).
Example:

Q4. What kind of elements form anions? Support your answer by two example.
Ans: Non-metals or electronegative elements whose atoms can gain one or more electrons form negative ions (or anions).
Examples:

Q5. Sodium atom reacts vigorously with water, but sodium ion does not. Why?
Ans: We know that the sodium atom (atomic number 11) has the electronic configuration 2,8,1. So, the sodium atom can lose one electronic from its outer shell to attain stable configuration. But the sodium ion (Na+ with a net charge of +1) has the stable electronic configuration (2,8).
That is why sodium atom reacts vigorously with water but sodium ion does not.
Q6. What is an inoic bond? State out at least three condition for its formation.
Ans: The electrostatic force of attraction which holds the two oppositely charged ions together is called the ionic bond.
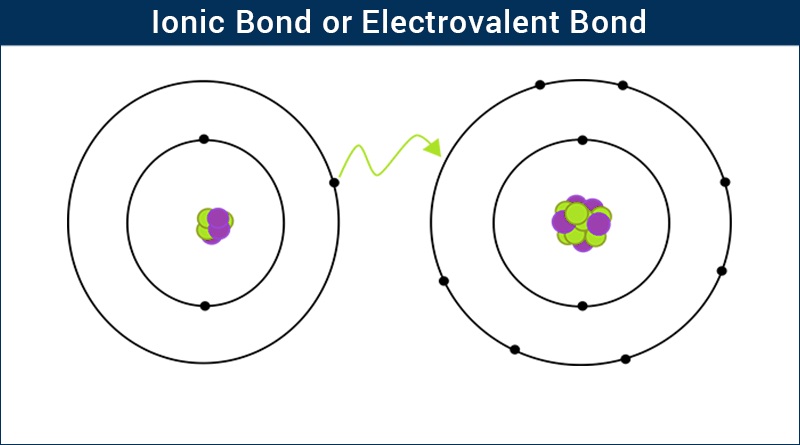
Ionic Bond (Electrovalent Bond) – Electrostatic Attraction between Oppositely Charged Ions
The Condition required for the formation of an ionic bond between two Elements are:
• Two elements must be of opposite charge.
• An element must have its less Ionisation Enthalpy(Cation).
• Another element must have high negative electron gain enthalpy(Anion).
• High lattice energy of the compound formed.
Q7. A metal A (atomic number 19 ) burns in chloride to produce a solid chloride ACl. By means of diagrams, show the arrangement of electrons in A before and after the reaction. Ans: The Ilustrated diagram using dots and cross is given below:

Q8. Give reason of the following:
a) Ionic compounds have high melting and boiling points.
b) Ionic compounds are good conductors of electricity in fused state or aqueous solution.
Ans: a) this is due to the strong electronic force of attraction between the oppositely charged ions. As a result, alarge amount of energy is needed to break the bond. That is why ionic compounds have high melting and boiling points.
b) This is because when they are method or dissolved in water, the ions become free to move. Hence they conduct electricity. That is why the ionic compounds are good conductors of electricity in fused state or aqueous solution.

Post a Comment
Post a Comment